Blood cells are derived from a common set of hematopoietic stem cells, which differentiate into more specific progenitors of the myeloid and lymphoid lineages, ultimately leading to differentiated cells. This developmental process is controlled by a complex regulatory network involving cytokines and their receptors, transcription factors and chromatin remodelers.
Based on public and novel data from molecular genetic experiments (qPCR, western blots, EMSA), along with genome-wide assays (RNA-seq, ChIP-seq), we defined a logical model recapitulating cytokine-induced differentiation of common progenitors, the effect of various reported gene knock-downs, as well as reprogramming of pre-B cells into macrophages induced by ectopic expression of specific transcription factors.
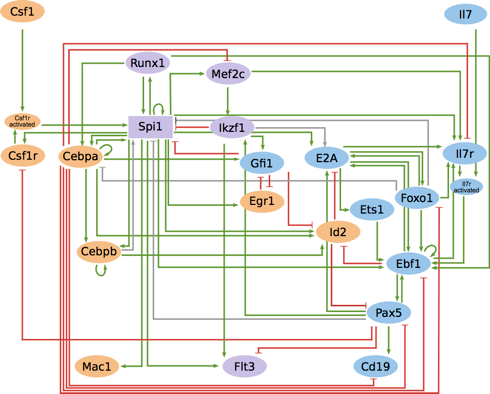
Note: This model is also available at BioModels database BioModels ID: 1610240000.